Secondary Battery Applications
Part of Chapter Battery Systems
ENGINE STARTING & SLI
1.3.1
Service: SLI stands for Starting/Lighting/Ignition – which is the ubiquitous automotive battery. Since these batteries are optimized for long float life, high peak currents, and infrequent shallow discharges, they would be a poor choice for powering an electric vehicle. They’re designed with thin plates (for the high peak current) and either free or AGM (absorbed) electrolyte. They’re not intended for cycling service.
As with all lead-acid batteries, high temperatures shorten their life.
EMERGENCY LIGHTING & ALARMS
1.3.2
Service: Long float; infrequent, moderate rate discharges (which may be shallow or full, but must be capable of 1.5 hours “run time” for emergency lighting as mandated by NFPA). They are not intended for cycling. Except for those infrequent discharges, they spend their entire lives on float charge at room temperature or above.
MOTIVE POWER
1.3.3
Service: Frequent full discharge and recharge cycling. In large enterprises, batteries may have two full cycles per day to satisfy the demands of three-shift operation. Their discharge profile ranges from low to high discharge rates. They must have high cycle life. They’re usually flooded cells, except for batteries used in small trucks such as golf carts, which are usually gelled or absorbed electrolyte. Motive power batteries are usually used at room or slightly elevated temperatures. Subject to mechanical shocks during service and maintenance.
STATIONARY
1.3.4
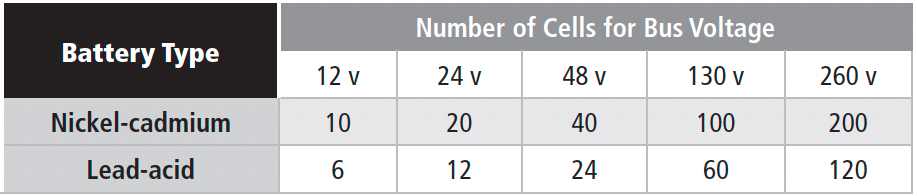
Service: Long float life, with infrequent moderate-to-deep discharge at variable rates. This design needs a compromise between long float life and high cycle life. Designed for use in a permanent location. Stationary batteries come in many flavors, from nickel-iron and NiCd to lead-acid of various constructions (flooded and sealed). There are also several standard bus voltages in use. Table 1c provides a quick rundown of the nominal number of cells needed for each bus voltage. They’re based on 1.2 VPC for NiCd and 2.0 VPC for lead-acid. You may see differences, though, especially for NiCd, because of their high equalize voltage requirements.
Remember that 1.2 VPC and 2.0 VPC are strictly nominal voltages, and that the open-circuit voltage of a lead-acid battery depends on its specific gravity. For more on this, see Float & Equalize Voltages: How Do They Differ? in SECTION 1.5.3.